Catalog
Learning Objectives:
Attendees will understand the benefits of ELISpot/FluoroSpot technology and their wide applications during drug development
Attendees will be able to describe challenges with sample preparation, assay design and implementation and identify approaches to manage them
Attendees will appreciate how new developments have improved ELISpot/FluoroSpot assay performance and recognize current limitations with the technology
About this item
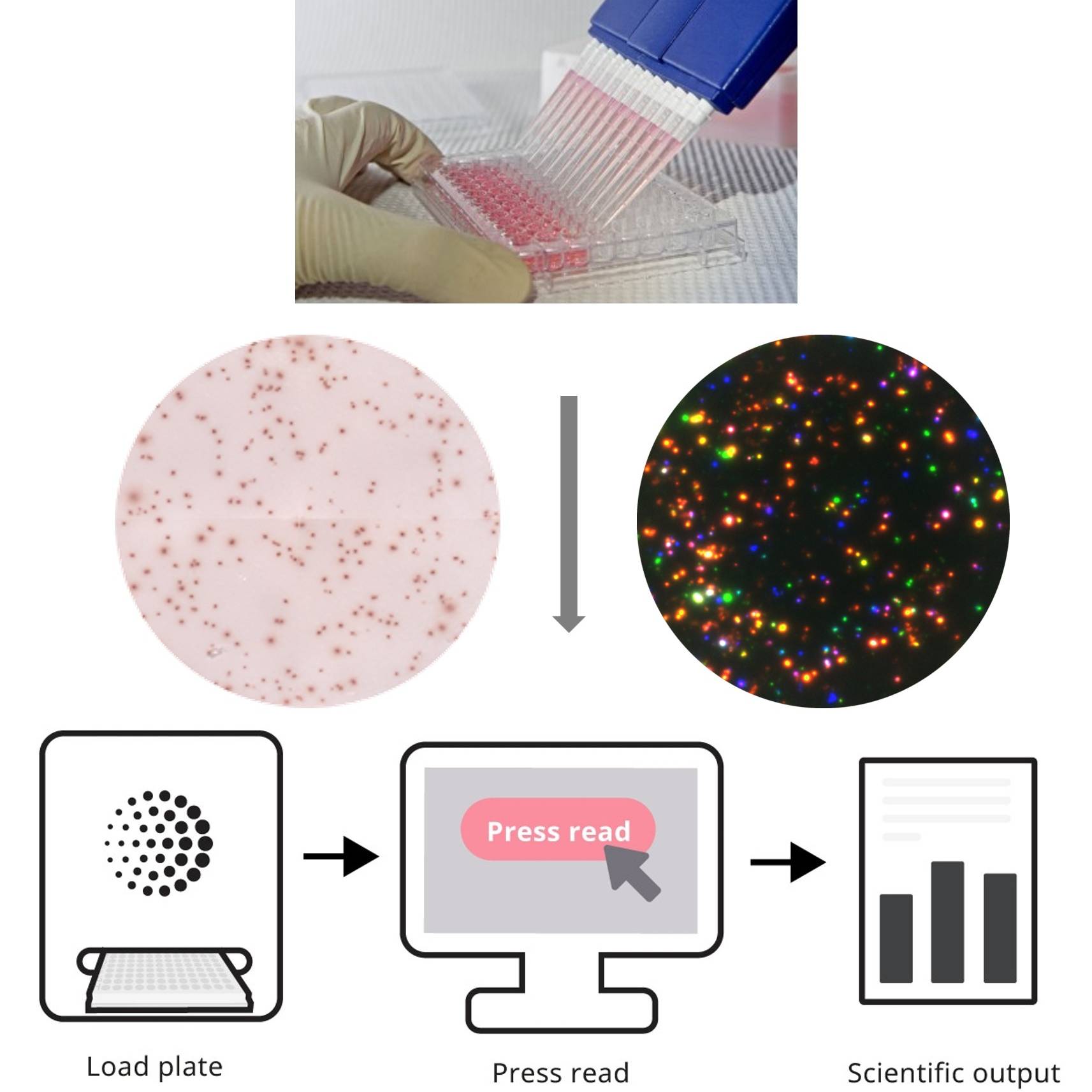
The ELISpot assay is a time-honored method for assessing the functionality of distinct immune cells in response to specific stimuli. It offers a simple format and is more sensitive and quantitative than other technological platforms. It is also broadly applicable, utilized for immunogenicity and biomarker assessments. Recent advancements have facilitated better standardization in methodology and data analysis which have streamlined the ELISpot workflow and enhanced the performance of this assay. This webinar will present foundational information about ELISpot and dive into these recent advancements. Case studies for common applications of this technology to measure cellular immune responses in various contexts throughout drug development will be presented. Gained knowledge about best practices for ELISpot testing, including key considerations for managing pre-analytical factors that impact sample quality, will be shared. Finally, critical challenges that can be encountered during implementation will be highlighted. This webinar seeks to enhance the audience’s knowledge of this assay platform and illustrate ways to apply this reliable technology to their drug development programs.
Learning Objectives:
- Attendees will understand the benefits of ELISpot/FluoroSpot technology and their wide applications during drug development
- Attendees will be able to describe challenges with sample preparation, assay design and implementation and identify approaches to manage them
- Attendees will appreciate how new developments have improved ELISpot/FluoroSpot assay performance and recognize current limitations with the technology
Speaker Information
Sylvia Janetzki, MD
Sylvia Janetzki, MD, has dedicated the past 26 years as President and CEO of ZellNet Consulting to advancing immune monitoring approaches for clinical studies with specific focus on Elispot and sample integrity. In a tight collaboration with the Cancer Research Institute, she established international proficiency panels for Elispot, Multimer staining, and ICS. These efforts led to the publication of assay harmonization guidelines ensuring consistency and reliability across studies. Sylvia is an integral part of the MIATA core team that promotes structured and transparent reporting of immune monitoring results. Her career includes numerous publications, book chapters and a comprehensive book on Elispot. Sylvia earned her MD from Humboldt University, Berlin, and completed her post-doctoral training in Immunology at Fordham University and MSKCC.
Xinyuan (Nemo) Li, Ph.D.
Xinyuan (Nemo) Li, Ph.D., is a Principal Scientist at Johnson & Johnson Innovative Medicine. He earned his Ph.D. in Pharmacology from Temple University and completed his postdoctoral training at the University of Pennsylvania, bringing 15 years of experience focusing on immunology research. In his current role, he oversees pre-clinical and clinical assessments of ELISpot/FluoroSpot assays across multiple modalities, including biologics, gene therapy, cell therapy, and therapeutic vaccines.
Madhan Masilamani, Ph.D.
Dr. Madhan Masilamani is a Scientific Associate Director at Bristol Myers Squibb, Princeton. His current work includes PK/ADA bioanalysis of biologics and CAR-T cells; and representation in cross-functional teams.
Madhan has >20 years of post-PhD experience in Immunology, Cell Biology and Molecular Biology. He has served in various SME roles both in Academia and Industry. He previously held Bioanalytical/DMPK roles at Boehringer Ingelheim and served as an Associate Professor of Pediatric Allergy & Immunology at Mount Sinai Medical Center.
Madhan received MSc in Medical Biochemistry from Pondicherry University, India and PhD in Biology/Immunology from University of Konstanz, Germany. He completed post-doctoral training at the National Institute of Allergy and Infectious Diseases, NIH, Bethesda. He has published 35 peer-reviewed articles, 8 reviews and 3 book chapters.
Karen Quadrini, Ph.D. (Moderator)
Karen J. Quadrini, Ph.D. has over 15 years of industry experience in biomarkers and translational research. She currently serves as Director of Clinical Biomarkers at Prothena Biosciences, where she is responsible for biomarker strategy and implementation, including assay development and validation, across the company’s portfolio. Prior to joining Prothena Biosciences, she was in the Biomarkers and Precision Medicine group at Passage Bio focused on biomarker strategic planning and assay development to support clinical development of the company’s investigational gene therapy products. Previously, she held roles of increasing responsibility in R&D at IRX Therapeutics (now Brooklyn Immunotherapeutics) and ICON Laboratory Services, where she provided predominantly tissue- and cell-based assay development support across multiple therapeutic areas. Dr. Quadrini received her Ph.D. in Biomedical Sciences from the Icahn School of Medicine at Mount Sinai, formerly Mount Sinai School of Medicine of New York University (NYC). She is an active volunteer for the American Association of Pharmaceutical Scientists (AAPS) and has been a member of the leadership team for the AAPS Biomarkers and Precision Medicine Community since 2019.
Sofie Pattyn (Moderator)
Sofie Pattyn (CTO and founder, Iqvia Laboratories, In Vitro Immunology formerly known as ImmunXperts) has over 25 years of experience in the field of immunogenicity assessment (vaccines and biotherapeutics) and in vitro assay development with a focus on functional assays for immunogenicity, immune-oncology and Cell and Gene Therapy products. She has extensive hands-on lab experience and has managed and coached several In Vitro teams over the last decade. From 2008 till 2013 she was Head of the In Vitro Immunogenicity group at AlgoNomics (Ghent, Belgium) and Lonza Applied Protein Services (Cambridge, UK). Prior to that, she worked at Innogenetics, Belgium for over 15 years.